Bianca Maria Piraccini
1. Dermatology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna
2. Department of Medical and Surgical Sciences University of Bologna, Italy
The management of androgenetic alopecia (AGA) is based in female on topical minoxidil and in men on oral finasteride and topical minoxidil, both of which are US FDA-approved treatment options; however, several off-label treatments for this condition are available.
(Saceda-Corralo D, Domínguez-Santas M, Vañó-Galván S, Grimalt R. What's New in Therapy for Male Androgenetic Alopecia? Am J Clin Dermatol. 2023 Jan;24(1):15-24. doi: 10.1007/s40257-022-00730-y. Epub 2022 Sep 28. PMID: 36169916).https://pubmed.ncbi.nlm.nih.gov/36169916/
Oral minoxidil
The possible oral administration of minoxidil in association or substitution of the local solution has been one of the major breakthroughs in the treatment of AGA in the last years.
Oral minoxidil (OM) is approved as an antihypertensive, with doses ranging between 10 and 40 mg daily. Low dose OM (LDOM) is not FDA-approved for treatment of AGA but is now worldwide prescribed at low dose for treating both male and female patients with AGA.
The drug is available in some countries as a 2.5 – 5 mg tablets, which can be cut in halves or quarters to achieve optimal dosage or is available as minoxidil powder that can be organized in capsules of the prescribed dose by a pharmacist.
LDOM (<5 mg daily) have been shown effective and safe for treating hair loss by several studies, not all focused on AGA, but also on other types of alopecia, including chronic telogen effluvium, traction alopecia, loose anagen syndrome, alopecia areata, monilethrix, chemotherapyinduced hair loss, and even scarring alopecia. The effectiveness of oral minoxidil is proportional to the dose taken by the patient.
Several articles have shown its efficacy and tolerability in AGA with different dosages.
In female AGA suggested dose ranges from 0.25 to 1.25 mg daily.
(Ramírez-Marín HA, Tosti A. Role of Oral Minoxidil in Patterned Hair Loss. Indian Dermatol Online J. 2022 Oct 12;13(6):729-733. doi: 10.4103/idoj.idoj_246_22. PMID: 36386734; PMCID: PMC9650732.)
https://pubmed.ncbi.nlm.nih.gov/36386734/
The daily dose may be divided in 2 or taken in a single administration, with satisfactory results and a safe profile. The author (BMP) utilizes the dose of 1 mg/day, often combined with spironolactone 25 mg in order to limit fluid/sodium retention side effects.
In male AGA, low dose OM is generally prescribed at doses ranging from 2.5 to 5 mg/day.
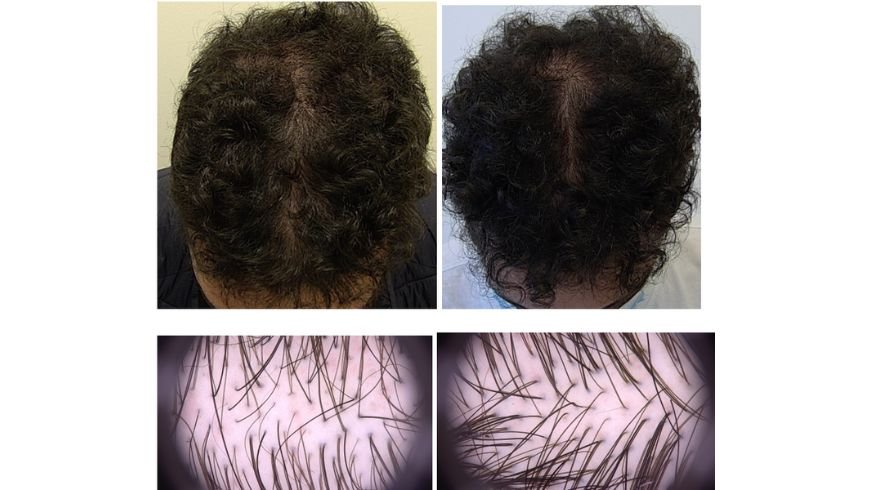
Result of therapy can be initially appreciated as soon as after 3 months of treatment and are visible after 6 months (Figure 1) with maximum effects after 12-15 months of administration.
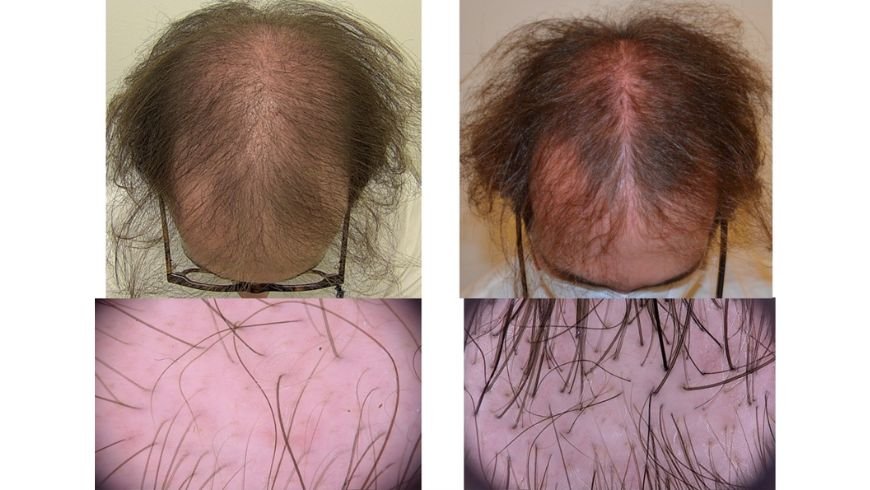
Figure 1 clinical and trichoscopy pictures of a 46-year-old male patients with moderate AGA before and after 6 months of minoxidil 2,5 mg/day administration.